Walther Nernst
1864 - 1941
Memorial website
Biography
(Nobel Foundation) Curriculum vitae (1890-02-06, in Latin)
Walther Hermann Nernst (article in
the local newspaper Göttinger Tageblatt, 2002-11-27, in German )
Chronology
Walther Nernst Museum, University of
Göttingen, physicochemical institute
Colloquium on Physical
Chemistry, University of Göttingen, Walther Nernst lecture hall
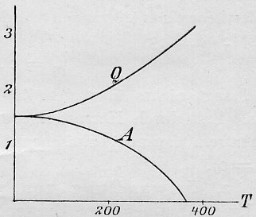 Original formulation of Walther Nernst:
Original formulation of Walther Nernst:
lim dA/dT = lim dU/dT = 0 for T
= 0
(Eq. 8 of Ref. 1, Eq. 4 of Ref. 2; however, Nernst used the symbol Q instead of U.
Left: Fig. 2 of Ref. 2)
The entropy change in a reaction between pure substances approaches zero at T
= 0. Nernst himself, however, did not make explicit use of the term entropy.
Max Planck subsequently (1910) made the stronger statement:
Beim Nullpunkt der absoluten Temperatur besitzt die Entropie eines jeden chemisch
homogenen Körpers von endlicher Dichte den Wert Null. [3]
(As the temperature diminishes indefinitely, the entropy of a chemically homogeneous
body of finite density approaches indefinitely near to the value zero).
S = 0 for T = 0
As a consequence, it follows from the Third Law that the absolute zero of the
(absolute) temperature scale, i.e., a temperature of zero kelvin (T = 0 K) cannot
be attained by any means.
This principle of the unattainability of the absolute zero has been formulated by
Fowler and Guggenheim as follows: "It is impossible by any procedure no matter how
idealized to reduce the temperature of any system to the absolute zero in a finite number
of operations" (R. H. Fowler and E. A. Guggenheim, Statistical Thermodynamics,
Cambridge University Press, 1940, p. 224).
For a detailed discussion of the different formulations of the Third Law see for
instance K. Denbigh, The Principles of Chemical Equilibrium, chapter 13.
Walther Nernst presented his theorem December 23, 1905 at a meeting of the Königliche
Gesellschaft der Wissenschaften zu Göttingen (Royal Academy of Sciences) [1] and
December 20, 1906 at a meeting of the Königlich Preussische Akademie der
Wissenschaften (Berlin) [2].
In 1921 the Nobel Prize in Chemistry
1920 was bestowed upon him "in recognition of his work in thermochemistry".
References
[1] W. Nernst. Ueber die Berechnung chemischer Gleichgewichte aus thermischen
Messungen. Nachr. Kgl. Ges. Wiss. Gött., 1906, No. 1, pp. 1 - 40 (facsimile).
[2] W. Nernst. Über die Beziehung zwischen Wärmeentwicklung und maximaler Arbeit bei
kondensierten Systemen. Ber. Kgl. Pr. Akad. Wiss., 1906, No. 52, pp. 933 - 940
[3] M. Planck. Vorlesungen über Thermodynamik. Veit, Leipzig, 3rd ed. (1911), ..., 5th
ed. (1917)
[4] F. Simon. Fünfundzwanzig Jahre Nernstscher Wärmesatz. Ergebn. exakt. Naturwiss.,
Vol. 9, pp. 222 - 274 (1930)
[5] R. Haase and W. Jost. 50 Jahre Nernstscher Wärmesatz. Naturwiss. 43
(1956) 481 - 486 (an excellent article on this subject)
1905/1906 - 2005/2006 Centennial
2006-11-09 Lecture
at the Georg August University, Göttingen
2006-10-05 Lecture
at the PTB, Braunschweig
2005-07-08 Colloquium at the
Humboldt University, Berlin
Nernst Equation (1888)
The Nernst equation describes the concentration dependence of the electromotive force
of an electrochemical cell, e.g., for a concentration cell (E0 = 0):
E = E0 - (R T / z F) ln (c2
/ c1)
(one better uses activities a instead of concentrations c)
This is of great importance, for instance, for nerve conduction, where the
potential difference of about 70 mV across the membrane (in the resting state) is the
result of correspondingly different concentrations of potassium ions (K+).
Nernst Distribution Law (1891)
If two liquids (or solids) a and b are partially immiscible and if
there is a third component i present in both phases which behaves individually as
an ideal solute (if it is sufficiently dilute), the ratio of its concentrations x
is independent of the individual values of x
xia / xib
= const.

Nernst lamp, AEG model A
More details ... |
Nernst Lamp (Nernst Glower) (1897)
The Nernst glower exhibits a bright emission in the visible spectrum (white light), and
it was initially used for ordinary electric illumination. The Nernst lamp (patented 1897)
could be operated in ambient air, whereas the carbon filament lamp of Thomas Edison (1879)
required a vacuum environment which was quite a disadvantage. In addition, the Nernst lamp
was twice as efficient and its light was more natural.
Emil Rathenau
who had already (1882) acquired the patent of Edison for the AEG (Allgemeine
Elektricitäts-Gesellschaft, the German General Electric Company, which soon became
the largest German electrical enterprise) also bought the patent
of Nernst (1898). At the World's Fair (World Exhibition) 1900 in Paris where the AEG
pavilion was illuminated by 800 Nernst lamps, W. Nernst received the Grand Prix (cf. Phys.
Z. 2 (1901) 300).
More on the Nernst lamp ...
Lambda Sensor and Solid Oxide Fuel Cell
Closely related to the Nernst lamp are the Lambda
Sensor and solid oxide fuel cells (SOFC) which utilize quite the same solid state
electrolyte. |
Photo-Induced Chain Reaction (1918)
Walther Nernst suggested a chain reaction mechanism for the photo-induced reaction of
chlorine with hydrogen in order to explain the large value of the quantum yield.
Cl-Cl + hv = 2 Cl
Cl + H-H = H-Cl + H
H + Cl-Cl = H-Cl + Cl
Cl + Cl + M = Cl-Cl + M
(M is a third body to carry away excess energy; this may also be the
wall.)
References
W. Nernst. Zur Anwendung des Einsteinschen photochemischen
Äquivalentgesetzes. I. Z. Elektrochem. 24 (1918) 335 - 336
Lotte Pusch. Zur Anwendung des Einsteinschen photochemischen Äquivalentgesetzes. II. Z.
Elektrochem. 24 (1918) 336 - 339
W. Nernst. Zur Kenntnis der photochemischen Reaktionen. (Nach Versuchen von W. Noddack.)
Phys. Z. 21 (1920) 602 - 605
Rudolf Göhring. Über den Mechanismus der photochemischen
Chlorknallgasreaktion und die Frage der Abklinggeschwindigkeit des durch das Licht
aktivierten Chlors. Z. Elektrochem. 27 (1921) 511 - 518
Ettingshausen-Nernst Effect (1887)
When a conductor or semiconductor is subjected to a temperature gradient and to a
magnetic field perpendicular to the temperature gradient, an electric field arises
perpendicular to both the temperature gradient and the magnetic field.
Ph.D. thesis of Walther Nernst. Collaboration with Albert von Ettingshausen, Graz,
following a suggestion of Ludwig Boltzmann. Continued in Würzburg at the laboratory of
Friedrich Kohlrausch.
Remarks: Sometimes the order of the names is reversed, i.e., Nernst-Ettingshausen
Effect; frequently the name of A. v. Ettingshausen is misspelled as Ettinghausen. Closely
related are the Ettingshausen Effect and the Nernst Effect.
Nernst Law of Electrical Nerve Stimulus Threshold (Reizschwellengesetz)
(1899)
[...]; nach unserer Theorie muß also die Stromintensität, die gerade noch einen Reiz
ausübt, mit der Quadratwurzel aus der Schwingungszahl direkt proportional ansteigen.
(Nernst, 1899)
(The minimum current that causes a stimulation increases proportionally to the square
root of the frequency.)
This law was supposed to hold for alternating currents of short duration. It is based
on the hypothesis that an electric current causes displacements of ions and related
changes in concentrations close to the cell membranes which act as reversible electrodes.
A fixed amount of electrical energy is required to cause a nerve stimulation. A closely
related law was derived for short pulses of direct currents. (Nernst, 1908)
const. = i × t½
Accommodation (Akkommodation)
Deviations from the law for longer times (the minimum current becomes significantly
larger than the law predicts) and the phenomenon that a stimulus can occur even when the
circuit is opened are explained by "some kind of accommodation" ("eine
Art Akkommodation") (Nernst, 1908).
Subsequently it turned out that Nernst's simple laws were not as general as he had
hoped. (Eucken and Miura, 1911)
R. v. Zeynek. Ueber die Erregbarkeit sensibler Nervenendigungen durch Wechselströme.
Nachr. Kgl. Ges. Wiss. Gött., 1899, No. 1, pp. 94 - 103 (facsimile)
W. Nernst. Zur Theorie der elektrischen Reizung. Nachr. Kgl. Ges. Wiss. Gött., 1899, No.
1, pp. 104 - 108 (facsimile)
W. Nernst. Zur Theorie des elektrischen Reizes. Pflügers Archiv ges. Physiologie 122
(1908) 275 - 314; DOI: 10.1007/BF01677956
A. Eucken and K. Miura. Zur Nernst'schen Theorie der elektrischen Nervenreizung. Pflügers
Archiv ges. Physiologie 140 (1911) 593 - 608;
DOI: 10.1007/BF01680585
The Neo-Bechstein piano was a modified acoustic grand piano using pick-ups to capture
naturally produced sound and subject it to electronic modification and amplification. It
was invented and designed by Nernst in 1930, together with the companies Bechstein (mechanical parts) and Siemens (electrical parts). The sound of the instrument
resembles that of an electric guitar rather than an acoustic piano. About 15 - 20
(or perhaps up to about 150, according to another source) instruments were built
of which about five are still in existence. However, only two are
still functioning; one is at the Technisches Museum Wien
(Vienna Museum of Technology), the other one is at Berlin.
Recently, the latter was played in the following performances:
2002-10-26 Kryptonale-8, Berlin;
2003-04-05 Podewil x-tract, Berlin; 2004-02-08 ZKM, Karlsruhe;
2004-03-26 MaerzMusik, Berlin.

W. Nernst, working on a single string model of the electro-acoustic
piano (Neo-Bechstein).
F. W. Winckel. Das Radio-Klavier von
Bechstein-Siemens-Nernst. Die Umschau 35 (1931) 840 - 843 (with 2
photographs)
C. Bechstein. Die Resonanz der Presse über den
neuen Bechstein-Siemens-Nernst Flügel (with 1 photograph)
Carl Bechstein. Een universeele vleugel
(in Dutch, for the exhibition "Klank en beeld", Amsterdam, May 6 - 16, 1932)
Photograph of W. Nernst tuning the Neo-Bechstein (1934)
The Scientific Monthly 56 (1942) 84
Neo-Bechstein. Kryptonale-8
(2002)
Peter Donhauser. Elektrische Klangmaschinen. Die Pionierzeit in Deutschland und
Österreich. Böhlau, Wien (2007), ISBN 3-205-77593-7
Neo-Bechstein.
Radiomuseum (2007)
Bibliography
Bibliography of Walther Nernst and his coworkers
Work in progress. So far about 870 references. Download nernst-bib.pdf
(PDF format, 199 KB, 36 pages)
Books
Walther Nernst. Theoretische Chemie vom Standpunkte der Avogadro'schen Regel und der
Thermodynamik. Enke, Stuttgart (1893)
W. Nernst and A. Schönflies. Einführung in die mathematische Behandlung der
Naturwissenschaften. Kurzgefasstes Lehrbuch der Differential- und Integralrechnung mit
besonderer Berücksichtigung der Chemie. München (1895)
F. Pollitzer. Die Berechnung chemischer Affinitäten nach dem Nernstschen
Wärmetheorem. Enke, Stuttgart (1912)
W. Nernst. Die theoretischen und experimentellen Grundlagen des neuen Wärmesatzes.
Knapp, Halle (1918)
Articles
Walther Nernst. Ueber die electromotorischen Kräfte, welche durch den Magnetismus in
von einem Wärmestrome durchflossenen Metallplatten geweckt werden. Inauguraldissertation,
Universität Würzburg. Annalen der Physik und Chemie N. F. 31 (1887) 760
- 789 (facsimile)
doi:10.1002/andp.18872670815
 Walther Nernst. Das Institut für Physikalische Chemie
und besonders Elektrochemie an der Universität Göttingen. Festschrift zur
Einweihungsfeier am 2. Juni 1896.
(facsimile)
Walther Nernst. Das Institut für Physikalische Chemie
und besonders Elektrochemie an der Universität Göttingen. Festschrift zur
Einweihungsfeier am 2. Juni 1896.
(facsimile)
Also Z. Elektrochemie 2 (1896) 629 - 636
Walther Nernst. Die Ziele der physikalischen Chemie.
Inauguration speech 1896-06-02, 18 pp.
(facsimile)
Walther Nernst. Die Nernst-Lampe. In: Mutter
Erde. Technik, Reisen und nützliche Naturbetrachtung in Haus und Familie. Verlag von W.
Spemann, Berlin, 1899. Zweiter Band, pp. 192 - 193. Nernst's
elektrische Glühlampe. Ibid., pp. 367 - 369
W. Nernst. Ueber die Bedeutung elektrischer Methoden für die
Chemie. Verh. Ges. Dt. Naturf. u. Ä. 73/1 (1901) 83 - 99
Friedrich Dolezalek. Das Institut für physikalische Chemie.
Festschrift (1906)
W. Nernst. Ueber die Berechnung chemischer Gleichgewichte aus thermischen Messungen.
Nachr. Kgl. Ges. Wiss. Gött., 1906, No. 1, pp. 1 - 40 (facsimile).
W. Nernst. Die Entwicklung der allgemeinen und physikalischen
Chemie. Ber. Dt. Chem. Ges. 40 (1907) 4617 - 4626
W. Nernst and J. Sand. Das Physikalisch-Chemische Institut an der Universität Berlin.
Z. Elektrochemie 15 (1909) 229 - 232
Ground-plan (590 KB)
W. Nernst and J. Sand. Das physikalisch-chemische Institut.
In: Max Lenz, Geschichte der Königlichen Friedrich-Wilhelms-Universität zu Berlin,
Verlag der Buchhandlung des Waisenhauses, Halle (1910)
Walther Nernst. Zum Gültigkeitsbereich der Naturgesetze.
Rede zum Antritt des Rektorates der Friedrich-Wilhelms-Universität in Berlin am 15.
Oktober 1921
Walther Nernst. Ueber das Auftreten neuer Sterne. Rede zur
Gedächtnisfeier des Stifters der Berliner Universität König Friedrich Wilhelms III in
Berlin am 3. August 1922
Obituaries
Robert A. Millikan. Walther Nernst, a great
physicist, passes. The Scientific Monthly 54 (1942) 84 - 86
Albert Einstein. The work and personality of Walther
Nernst. The Scientific Monthly 54 (1942) 195 - 196
Biographies
Günther Bugge. Walter Nernst. Zum 50. Geburtstag am
25. Juni [1914]. Reclams Universum-Jahrbuch 1914, Nr. 23, pp. 257 - 259
Werner Haberditzl. Walther Nernst. In: Gerhard
Harig (ed.). Von Adam Ries bis Max Planck. 25 große deutsche Mathematiker und
Naturwissenschaftler. VEB Verlag Enzyklopädie, Leipzig, 1961. 2nd ed., 1962. pp. 118 -
122.
Also in: Gerhard Harig (ed.). Deutsche Forscher aus sechs Jahrhunderten. Lebensbilder von
Ärzten, Naturwissenschaftlern und Technikern. VEB Bibliographisches Institut, Leipzig,
1965. pp. 373 - 377
Kurt Mendelssohn. The world of Walther Nernst. The rise and fall of german science.
Macmillan (1973)
German translation: Walther Nernst und seine Zeit. Aufstieg und Niedergang der deutschen
Naturwissenschaften (1976)
Hans-Georg Bartel. Walther Nernst. BSB B. G. Teubner Verlagsgesellschaft, Leipzig
(1989)
Diana Kormos Barkan. Walther Nernst and the Transition to Modern Physical Science.
Cambridge University Press (1998)
Hans-Georg Bartel and Rudolf P. Huebener. Walther Nernst: Pioneer of Physics
and of Chemistry. World Scientific, Singapore (2007)
Miscellanies
Alwin Mittasch. Geschichte der Ammoniaksynthese. Verlag Chemie, Weinheim (1951)
Lothar Suhling. Walther Nernst und die Ammoniaksynthese nach Haber und Bosch. In:
Helmuth Albrecht (ed.). Naturwissenschaft und Technik in der Geschichte. GNT-Verlag,
Stuttgart (1993)
http://www.gnt-verlag.de/programm/15/p343-356_suhling.shtml
Curiosities
Walther Nernst. Vita. (Transcription of W. Nernst's
curriculum vitae of 1890-02-06, written by hand in Latin when he applied for a position at
Göttingen university)
Walther Nernst and Lotte Warburg. Zwischen Raum und Zeit.
(1912) (A relativistic romance)
Züs Colonna (pseudonym of Lotte Warburg). Walther
Nernst. (1941?) (A satirical obituary)
Picture Gallery
Kohlrausch and coworkers, Würzburg 1887
Boltzmann and coworkers, Graz 1887
The Göttingen physicochemical institute founded by Nernst in
1895
W. Nernst, A. Coehn and coworkers 1903
Solvay Congress, Brussels 1911

c. 1900 |

Photograph by Nicola Perscheid 1906.
Photogravure published by
Photographische Gesellschaft Berlin |

c. 1924
Z. phys. Chem. 110 (1924) |

Retired, c. 1934
Photograph by his daughter Angela Hahn
Naturwiss. 22 (1934) 437-439 |

W. Nernst attending a colloquium
Drawing by Walther Adolf Roth 1905 or 1906
cf. Naturwiss. 36 (1949) 225-229 |

Painting by Max Liebermann 1912
(reproduction by Frisch)
signed by W. Nernst |

Etching by Hermann Struck 1921
No. 1/50
signed by the artist and by W. Nernst |
Autograph: Letter (envelope - front,
back) of W. Nernst to
H.
v. Euler-Chelpin, Stockholm, 1922-01-07
Photograph with a motto (Fortes fortuna adjuvat) 1924
Autograph with a motto (Numquam retrorsum!) 1931
Autograph: Letter of W. Nernst to I. Langmuir
1933-01-17 (congratulation to Nobel prize)
Walther Nernst and his cars
Walther Nernst on stamps (Germany 1950, Sweden 1980)
Related Persons
Abegg, Richard (1864 - 1910) Assistant of Nernst at Göttingen,
professor at Breslau
Abel, Emil ( - ) Student of Nernst. Founder of physics in Argentina (university of La
Plata)
Althoff, Friedrich (1839 - 1908) Omnipotent promoter of science at the Prussian
Ministry of Culture
Arrhenius,
Svante (1859 - 1927) Chemist
Auer von Welsbach, Carl (1858 - 1929) Inventor
Avogadro, Amedeo (1776 - 1856)
Bodenstein, Max (1871 - 1942) Professor in Berlin,
Hannover and again Berlin (successor of Nernst)
Boltzmann, Ludwig (1844 - 1906) Teacher of Nernst at Graz
Coehn, Alfred (1863 - 1938) Coworker of Nernst at Göttingen
Cremer, Erika (1900 - 1996) Student of Nernst and Bodenstein
Danneel, Heinrich (1867 - 1942) Student of Nernst at Göttingen
Dolezalek, Friedrich (1873 - 1920) Student of Nernst at
Göttingen, professor at Göttingen and Charlottenburg
Drude, Paul (1863 - 1906) Coworker of Nernst at Göttingen, professor at Berlin
Edison, Thomas Alva (1847 - 1931) Electrician
Einstein,
Albert (1879- 1955) Physicist
von Ettingshausen, Albert (1850 - 1932) Teacher of Nernst at Graz
Eucken, Arnold (1884 - 1950) Student of Nernst, professor at
Breslau and Göttingen
von
Euler-Chelpin, Hans (1873 - 1964) Post-doc with Nernst at Göttingen
Haber, Fritz
(1868 - 1934) Professor at Karlsruhe, director KWI
von Helmholtz, Hermann (1821 - 1894) President PTR 1887 - 1894
Kohlrausch, Friedrich (1840 - 1910) Teacher of Nernst at Würzburg. President PTR 1895
- 1905
Langmuir,
Irving (1881 - 1957) Student of Nernst at Göttingen
von Laue, Max
(1879 - 1960) Physicist
Lindemann, Charles (1884 - ?) Student of Nernst at Berlin
Lindemann, Frederick Alexander (1886 - 1957) (Viscount Cherwell) Student of Nernst at
Berlin
Loeb, Morris (1863 - 1912) Student of Ostwald and coworker of Nernst. Founder of
physical chemistry in the USA
Lohmeyer, Emma (1871 - 1949) Wife of W. Nernst
Maltby, Margaret E. (1860 - 1944) Student of Nernst at
Göttingen
Mendelssohn, Kurt (1906 - 1980) Student of Nernst at Berlin. Biographer
Meyer, Victor (1848 - 1893) Professor of chemistry at Göttingen and Heidelberg
Millikan,
Robert A. (1868 - 1953) Post-doc with Nernst at Göttingen
Ostwald, Wilhelm (1853 - 1932) Founder of physical chemistry,
professor at Dorpat and Leipzig, teacher of Nernst
Planck, Max
(1858 - 1947) Physicist
Rathenau,
Emil (1838 - 1915) Director AEG
Rathenau,
Walther (1867 - 1922) Politician
Riecke, Eduard (1845 - 1915) Professor of physics at Göttingen
Riesenfeld, Ernst Hermann (1877 - 1957) Student of Nernst
Roloff, Max (1871 - ?) Student of Nernst at Göttingen
Rubens, Heinrich (1865 - 1922) Predecessor of Nernst
Schönflies, Arthur Moritz (1853 - 1928) Professor at Göttingen and Königsberg
Simon, Franz (1893 - 1956) PhD student and coworker of Nernst; successor of Eucken at Breslau
Solvay, Ernest (1838 - 1923) Industrialist
Stark, Johannes
(1874 - 1957) Opponent of Nernst. Director PTR 1933 - 1939
Tammann, Gustav (1861 - 1938) Coworker of Nernst,
professor at Göttingen
Warburg, Emil (1846 - 1931) President PTR 1905 - 1922
Warburg, Lotte (1884 - 1948) (Züs Colonna)
Writer
von Wartenberg, Hans (1880 - 1960) Coworker of Nernst at
Göttingen and Berlin, professor at Danzig and Göttingen
Wehnelt, Arthur (1871 - 1944) Successor of Nernst
Westinghouse, George (1846 - 1914) Industrialist
Disclaimer: The owner and the webmaster of this site are not responsible for any
contents and links outside the nernst.de domain.
Impressum
Revised 2023-09-13 by Webmaster













